 Business Management
Business Management
 IT Services
IT Services
 Business Management
Business Management
 Business Analytics
Business Analytics
 Business Analytics
Business Analytics
 Business Analytics
Business Analytics
 Best Credit Cards
Best Credit Cards
 Best Credit Cards
Best Credit Cards
 Best Credit Cards
Best Credit Cards
Can a DBS be removed? Yes, a DBS (Deep Brain Stimulation) can be removed surgically if necessary. Learn more about the procedure, reasons, and implications in this blog.
The removal of a DBS system is a complex procedure that should only be performed by a skilled neurosurgeon. The decision to remove the system is usually made after a thorough evaluation of the patient's condition and discussions with the medical team. The reasons for removal can vary, but some of the common factors include infection, device malfunction, or if the benefits of the system no longer outweigh the risks or side effects.
Infection is one of the primary reasons for DBS system removal. Although rare, it can occur after the initial implantation or at any point during the time the system is in place. The symptoms of an infection may include fever, pain, redness, or drainage at the implant site. In such cases, it is crucial to remove the system to prevent the spread of infection to other parts of the brain.
Device malfunction is another factor that may require the removal of a DBS system. The DBS system consists of electrodes, a pulse generator, and connecting wires. If any of these components fail, it can lead to a loss of therapeutic effect or undesirable side effects. In such situations, removing the system and replacing the malfunctioning parts may be necessary.
Changes in the patient's condition can also lead to the removal of a DBS system. For example, if the patient's symptoms improve significantly, and medication adjustments are made, the system might no longer be needed. Additionally, if the patient develops new neurological conditions that are not compatible with the DBS system, removal may be the best course of action.
The removal procedure itself involves several steps. The first step is to make an incision and expose the area where the electrodes and pulse generator are located. The positioning and attachment of the electrodes make their removal a delicate process. The neurosurgeon must carefully disconnect the electrodes and remove them without causing damage to surrounding brain tissue.
Once the electrodes are removed, the pulse generator is next. It is usually located beneath the skin, often near the collarbone or abdomen. The incision is made in the appropriate location, and the neurosurgeon disconnects the generator from the connecting wires before removing it from the body.
Recovery after removal depends on the individual and the specific circumstances of the procedure. The incisions will need time to heal, and the patient may experience some discomfort or pain during the initial recovery period. The medical team will provide instructions on post-operative care and pain management.
It is important to note that the decision to remove a DBS system should not be taken lightly. It is a complex and irreversible procedure that requires careful consideration and consultation with the medical team. The benefits, risks, and alternatives should be thoroughly evaluated before making a final decision.
In conclusion, a DBS system can be removed, but it is a complex procedure that should only be performed by a skilled neurosurgeon. Reasons for removal may include infection, device malfunction, or changes in the patient's condition. The removal involves disconnecting and removing the electrodes and pulse generator. Recovery after removal varies for each individual. Ultimately, the decision to remove a DBS system should be made after careful consideration and consultation with the medical team.
Yes, a DBS (Deep Brain Stimulation) device can be removed. The removal procedure is typically performed if the device is no longer effective or if there are complications associated with it.
2. Is removing a DBS a major surgery?Removing a DBS device is considered a major surgery. It requires a skilled neurosurgeon and is performed under general anesthesia. The procedure involves locating and removing the implanted electrodes and the pulse generator.
3. What are the reasons for removing a DBS?A DBS device may be removed if it is no longer effective in controlling the symptoms of a neurological condition or if it causes significant complications such as infection, erosion of the skin, or hardware malfunction. In some cases, removal may also be necessary if the patient wants to switch to a different treatment option.
4. Are there any risks associated with removing a DBS?The removal of a DBS device carries certain risks, including the potential for infection, bleeding, and damage to surrounding brain tissue. In addition, there is a possibility of experiencing a return of symptoms after the device is removed.
5. Can a DBS be re-implanted after it has been removed?In some cases, a DBS device can be re-implanted after it has been removed. However, this decision is made on a case-by-case basis and depends on factors such as the patient's overall health, the reasons for the removal, and the availability of alternative treatment options.
 LATEST ARTICLES
LATEST ARTICLES

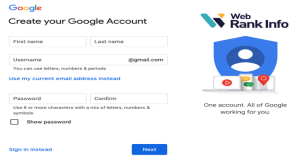
Are Google accounts free to make?

Are three types of strategies that organizations can use to adapt to enviro..

Can a single-member LLC add a second member later in Texas?

Can I do my masters in USA without GRE?

Can I be a data analyst if I'm bad at math?

Are two techniques used in descriptive analytics?

Are closed accounts good on your credit report?

Can a Visa card be used for gas?

Are user name and user ID the same?

Can I find my UTR number online?

Do I need to get my car inspected before registration in SC?

Am I at risk if someone has my bank statement?
 POPULAR ARTICLES
POPULAR ARTICLES

Are Google accounts free to make?

Are three types of strategies that organizations can use to adapt to enviro..

Can a single-member LLC add a second member later in Texas?

Can I do my masters in USA without GRE?

Can I be a data analyst if I'm bad at math?

Are two techniques used in descriptive analytics?

Are closed accounts good on your credit report?

Can a Visa card be used for gas?

Are user name and user ID the same?

Can I find my UTR number online?